Crude oil is one of the most important and versatile natural resources in the world. It serves as the primary raw material for many industries and is the foundation for producing gasoline, diesel, jet fuel, and countless petrochemical products. However, before crude oil can be transformed into these valuable products, it must first undergo a series of complex processes. One of the key processes is heating crude oil, a fundamental step in refining and separating its various components. But what exactly happens when crude oil is heated, and why is this heating so crucial in the production of valuable products? This article explores the physical and chemical changes that occur when crude oil is heated, and the critical role heat plays in its transformation.
The Composition of Crude Oil
Before diving into what happens when crude oil is heated, it’s important to understand what crude oil is composed of. Crude oil is a mixture of hydrocarbons, which are organic compounds made up of hydrogen and carbon atoms. These hydrocarbons can vary greatly in size, ranging from small molecules such as methane and ethane to large, complex molecules like asphaltenes and waxes. In addition to hydrocarbons, crude oil may contain small amounts of sulfur, nitrogen, oxygen, and trace metals such as vanadium and nickel.
These hydrocarbons in crude oil have a wide range of boiling points, which means that different components of crude oil vaporize at different temperatures. The ability to separate these components is key to refining crude oil into its usable products. Understanding this diversity in boiling points is essential for grasping the importance of heating crude oil during the refining process.
The Role of Heating in the Refining Process
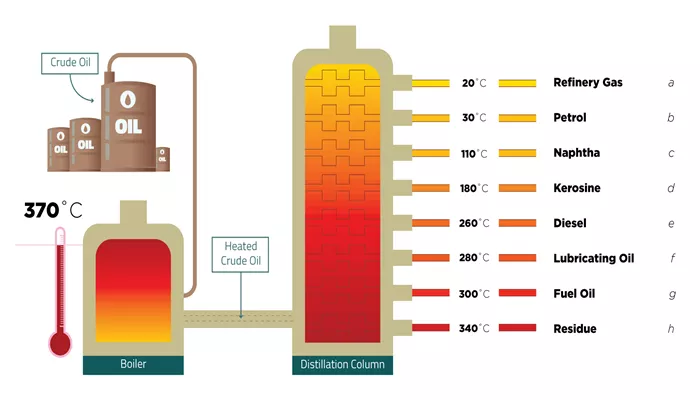
The primary goal of heating crude oil is to separate its various components based on their boiling points. When crude oil is heated, it undergoes a physical change that allows its different hydrocarbon components to vaporize at specific temperatures. This process is the basis for fractional distillation, which is one of the most crucial methods used in oil refineries to separate the different products that can be derived from crude oil, such as gasoline, diesel, kerosene, and heavy fuel oils.
Fractional distillation involves heating crude oil in a furnace until it begins to vaporize. As the vapor rises through a distillation column, it cools and condenses at different levels, with the lighter components condensing at the top and the heavier components condensing further down. This process allows for the separation of the various hydrocarbons in crude oil, each with its own boiling point.
The Process of Vaporization
As crude oil is heated, the temperature gradually increases, causing the different hydrocarbons to change from liquid to gas. The lighter hydrocarbons, such as methane, propane, and butane, have lower boiling points, so they vaporize at lower temperatures. Heavier hydrocarbons, like those found in diesel or lubricating oils, require higher temperatures to vaporize.
The temperature range at which each component of crude oil vaporizes is a critical aspect of refining, as it determines which products can be obtained from crude oil. For example, gasoline typically comes from hydrocarbons with boiling points ranging from 30°C to 200°C (86°F to 392°F), while diesel is made from heavier components with boiling points between 200°C and 350°C (392°F and 662°F). By carefully controlling the heating process, refineries can extract specific products from crude oil based on their boiling points.
The Role of Heat in Cracking
In addition to fractional distillation, heating crude oil also plays a key role in cracking, a process that helps break down larger, heavier hydrocarbons into smaller, more useful molecules. Cracking is essential for maximizing the yield of valuable products like gasoline from crude oil. There are two primary types of cracking: thermal cracking and catalytic cracking.
Thermal Cracking: Thermal cracking involves heating crude oil to very high temperatures, typically between 450°C and 750°C (842°F and 1,382°F), to break apart large hydrocarbon molecules. The high heat causes the molecular bonds to break, resulting in smaller molecules that can be used to make gasoline, diesel, and other lighter products. While thermal cracking is effective, it is less efficient than catalytic cracking and can produce undesirable byproducts, such as coke.
Catalytic Cracking: Catalytic cracking is a more efficient process that also involves heating crude oil but adds a catalyst to speed up the chemical reactions. The catalyst helps break down the larger molecules at lower temperatures and pressures, producing a higher yield of gasoline and other valuable products. Catalytic cracking is widely used in modern refineries to convert heavier fractions of crude oil into more desirable products.
Heating is thus not only used to separate the different components of crude oil through distillation but also to break down larger molecules into smaller, more useful ones through cracking. These processes are essential for refining crude oil into the fuel products that power the global economy.
The Chemical Changes That Occur When Crude Oil is Heated
In addition to the physical changes that occur when crude oil is heated, there are also chemical changes that take place. As crude oil is exposed to heat, the bonds between atoms in the hydrocarbons are weakened. This can lead to various chemical reactions, including cracking, polymerization, and oxidation.
Cracking: As mentioned earlier, cracking is a chemical process that occurs when the high heat applied to crude oil breaks the bonds between carbon atoms in the hydrocarbon molecules. This leads to the formation of smaller molecules, which are essential for producing gasoline, diesel, and other lighter hydrocarbons. The cracking process can be enhanced by using catalysts, which help speed up the chemical reactions and improve the efficiency of the process.
Polymerization: Polymerization is the process by which smaller molecules combine to form larger, more complex molecules. In crude oil, polymerization can lead to the formation of larger molecules that can be undesirable for the refining process. For example, polymerization can result in the formation of heavy waxes or asphalt, which are difficult to refine into usable products.
Oxidation: Oxidation occurs when hydrocarbons in crude oil react with oxygen during the heating process. This can result in the formation of acids, resins, or other undesirable byproducts. Oxidation can also lead to the degradation of certain components in crude oil, which can affect the quality of the final products.
The chemical changes that occur when crude oil is heated are an essential part of the refining process. These reactions help break down crude oil into its various components and allow for the creation of valuable products like gasoline, diesel, and jet fuel. However, the challenge for refiners is to control these chemical reactions to ensure that the desired products are obtained and that undesirable byproducts are minimized.
Environmental Considerations When Heating Crude Oil
Heating crude oil is an energy-intensive process that requires significant amounts of fuel. This has important environmental implications, as the refining process can generate greenhouse gas emissions and other pollutants. The use of fossil fuels to heat crude oil contributes to climate change, and the refining process itself can produce emissions such as sulfur dioxide, nitrogen oxides, and volatile organic compounds (VOCs), which can have harmful effects on air quality.
To mitigate these environmental impacts, the refining industry has been working to improve the energy efficiency of heating processes and reduce emissions. Advances in catalytic cracking, hydrocracking, and other refining technologies have helped reduce the environmental footprint of the industry. Additionally, the use of alternative energy sources and the implementation of cleaner technologies are crucial for reducing the environmental impact of heating crude oil.
The Importance of Heating Crude Oil
Heating crude oil is a fundamental process in the refining industry. By heating crude oil, refineries can separate its various components, break down larger molecules into smaller ones, and produce the valuable products that are essential to modern life. The processes of fractional distillation, cracking, and reforming are all heavily reliant on heat to transform crude oil into gasoline, diesel, jet fuel, and other petrochemical products.
However, heating crude oil also involves complex chemical reactions and environmental challenges. The refining industry continues to innovate and improve its technologies to maximize efficiency, reduce environmental impact, and meet the growing global demand for refined products. Understanding the science behind heating crude oil and the processes that occur during this transformation is essential for appreciating the complexity of the petroleum industry and the vital role that crude oil plays in powering our modern world.
Related Topics:
How Is Gasoline Separated from Crude Oil?
Which Country Will Have the Highest Crude Oil Output in 2025?